Improving the delivery of acute wound care services in hospitals
Wounds cause pain, discomfort and can compromise quality of life. They also place patients at risk of various complications such as deadly infections.
Our research improves the care provided to hospitalised patients with wounds, resulting in better patient experiences and outcomes, and savings to the health system. Almost all patients in hospital have a wound, whether it be a surgical wound, a wound from an intravascular device (drip) or a pressure injury.
To achieve our vision, we investigate how to prevent and treat these wounds and ensure this knowledge translates into both policy and practice.
Research highlights
Wiser wound care focus of new Centre of Research Excellence
Griffith University has been awarded a $2.5 million National Health and Medical Research Grant to develop a Centre of Research Excellence (CRE) to improve delivery of acute wound care services in hospital.
Our Mission
We undertake systematic reviews and implementation/de-implementation research to improve patient outcomes. We focus on engaging with end-users, both consumers and clinicians in our research and knowledge translation activities, as well as providing research training.
Focus areas
We focus on three high-volume, high-risk and high-cost acute wounds that occur in hospital.

Pressure injuries
Pressure injuries are potentially preventable injuries to the skin and underlying tissues. In hospitalised patients, pressure injury prevalence is 12.8%. Our research focuses on developing evidence-based interventions that increase hospitalised patients' involvement in prevention of pressure injuries, contributing to reducing the clinical and cost impacts on patients, their families and the healthcare system.
Experts: Professor Wendy Chaboyer, Professor Liz McInnes and Professor Brigid Gillespie
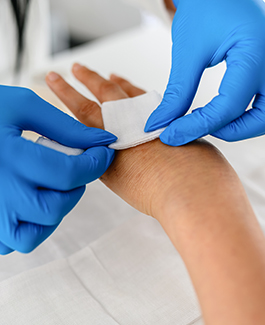
Surgical wounds
Postoperative wound infection is one of the most avoidable hospital-acquired complications. Between 11-19% of patients develop a postoperative wound infection up to 30 days following surgery. Our research is focused on developing interventions to inform improvements in the safety, quality and experiences of surgical patients and their families by generating high quality research evidence that promotes the best clinical practice.
Experts: Professor Brigid Gillespie and Professor Wendy Chaboyer
Intravascular devices
Intravascular device wounds/skin complications are extremely prevalent but preventable. Globally, 25% of central and 36% of peripheral IVDs are removed due to complications ( e.g. bruising, swelling, dermatitis). Our research focuses on developing evidence-based interventions that minimise complications and optimise patient care.
Experts: Professor Claire Rickard and Professor Amanda Ullman
Logos

NHMRC Centre of Research Excellence
The National Health Medical Research Council (NHMRC) awarded our team a 5-year Centre of Research Excellence (CRE - App1196436) in Wiser Wound Care. Our CRE was established on 1 January 2021.
Partner with us
Partnerships and collaboration are essential to our research impact. We partner widely with industry, not-for-profit organisations and government. We also collaborate with fellow researchers and maintain strong ties with our community.
If you would like to partner with us, or tap into our research expertise, facilities or services, please get in touch.
Research projects
Pressure injuries
- Use of prophylactic dressings to prevent sacral PI in adult medical/surgical patients (NHMRC $1.8 million; completed. See Gillespie B et al BMJ 2021:373 Article n93).
- Comparison of 2 prophylactic dressings to prevent sacral PI in adult ICU patients: a pilot RCT (MHIQ funding $17,000; data analysis).
- Testing the efficacy of two sacral pressings to prevent pressure injuries in adult intensive are patients (Queensland Health Innovation, Investment and Research Fellowship $117,000; soon to start recruiting).
- Use of subepidermal moisture scanning as a PI risk assessment strategy: a pilot RCT (CRE funding; completed. See Campbell et al J Tissue Viability 2022 (early view).
- Cochrane reviews of support strategies for PIP (completed. See Shi C et al Cochrane Database of Systematic Reviews 2021).
- Cochrane review update of nutritional interventions for preventing and treating pressure injuries (under review)
- Clinicians’ and patients’ views on the prevention and management of pressure injuries: a mixed-methods systematic review (ACU Faculty of Health Sciences Research Project Grant for ECR/MCR $7,853.44; revise manuscript for submission to IJNS)
- Clinical guidelines for nutritional and support surface interventions to prevent pressure injuries: a qualitative study of clinician views (SVHM Research Endowment Fund 2022 $19,443.56; completed data collection)
- Identifying priority barriers for an intervention to improve evidence-based pressure injury care from the perspective of clinical and consumer end-users: a qualitative study (ACU Research Award for Women Academic Staff $12,497.78; ethics preparation)
Surgical wounds
- Enhanced Recovery After Surgery (ERAS) national surveys of perioperative and surgical nurses, surgeons and anaesthetists
- Co-design study of ERAS implementation
- Patient experience in surgical care
- Development of a patient experience measure in surgical care
- Patient participation in surgical wound care following surgery
- Patient education interventions to improve patient outcomes
- Incidence and costs of surgical site infections and surgical wound dehiscence
Intravascular devices
- Peripherally InSerted Central catheter Securement: the PISCES Trial. (NHMRC $1.1M) (data analysis)
- Measurement and sequelae of neonatal mechanical skin injuries. (RBWH Foundation; $51,120). (recruitment under way)
- Improving intravenous catheter selection to reduce extravasations for children: an implementation study. (Nursing and Midwifery Research Fellowships, $119,943 (recruitment underway) and Children’s Hospital Foundation, $97,553 (data analysis).
- ivWatch® to prevent severe extravasation injuries in the Paediatric Intensive Care: a pilot, randomized controlled trial (Children’s Hospital Foundation, $81,292) (data analysis).
- Systematic review of implementation strategies for IV care (unfunded) (manuscript under review)
- Systematic review of extravasation management in paediatric and neonatal care (funded by UQ Summer Scholars) (accepted, J Hospital Medicine).
- Securing jugular central venous access devices with dressings fixed to a liquid adhesive in an intensive care unit population: a randomised controlled trial. (Eloquest Healthcare, investigator initiated grant, $148k, recruiting).
- “Protect PIVs”: A pilot randomized controlled trial of a novel antimicrobial dressing in peripheral intravenous catheters (PIVs). The ProP Trial. (3M, investigator initiated grant, $309k, at ethics).
- Dressings and securement devices to prevent complications for peripheral arterial catheters. Cochrane Review. Submitted.
- Antimicrobial dressings for central venous catheters (CVC). Proposal submitted.
- Adherence to the peripheral intravenous catheter (PIVC) GUIDEline for promoting evidence-based PIVC insertion and management in the Emergency Department (GUIDE-ED), PAH SERTA $100k).
Lead
Professor Wendy Chaboyer
Professor Chaboyer is an experienced nurse researcher who has been inducted into the Sigma Theta Tau International Nurse Researcher Hall of Fame and is a lifetime honorary fellow of the Australian College of Critical Care Nurses.
Chief Investigators
Associate Investigators
Research Fellows
Other team members
Senior Research Assistants
- Stephanie Hall
- Jonathan Vico Da Silva
Business Support Officer
- Helen Liu
Contact details
- Phone
- (07) 5678 8811
- mhiq@griffith.edu.au
- Media enquiries
- media@griffith.edu.au
- Location and postal address
- Menzies Health Institute Queensland
- G40 Griffith Health Centre, Level 8.86
- Gold Coast campus
- Griffith University QLD 4222